Category: Medications - Page 2
Cough Suppressants and MAOIs: What You Need to Know About Dextromethorphan Risks
Dextromethorphan in common cough medicines can cause life-threatening serotonin syndrome when taken with MAOI antidepressants. Learn the risks, safe alternatives, and what to do if you're on one of these drugs.

Recent Patent Cases and Generic Delays: 2023-2025 Examples
Between 2023 and 2025, FDA-approved generic drugs faced average delays of 3.2 years before reaching patients due to patent litigation. Learn how patent thickets, the 30-month stay, and complex generics are blocking access-and who’s paying the price.

Global Perspectives on Generics: How Countries Cut Drug Costs Without Compromising Care
Discover how countries around the world use generic drugs to cut healthcare costs-from China's aggressive bulk buying to the U.S.'s high-volume model-and why quality and sustainability are at risk as prices fall.
Opioids with MAOIs: Why This Combination Can Be Deadly and How to Avoid It
Combining opioids like tramadol or meperidine with MAOIs can cause fatal serotonin syndrome. Learn which opioids are dangerous, how long to wait after stopping an MAOI, and what pain relief is safe.
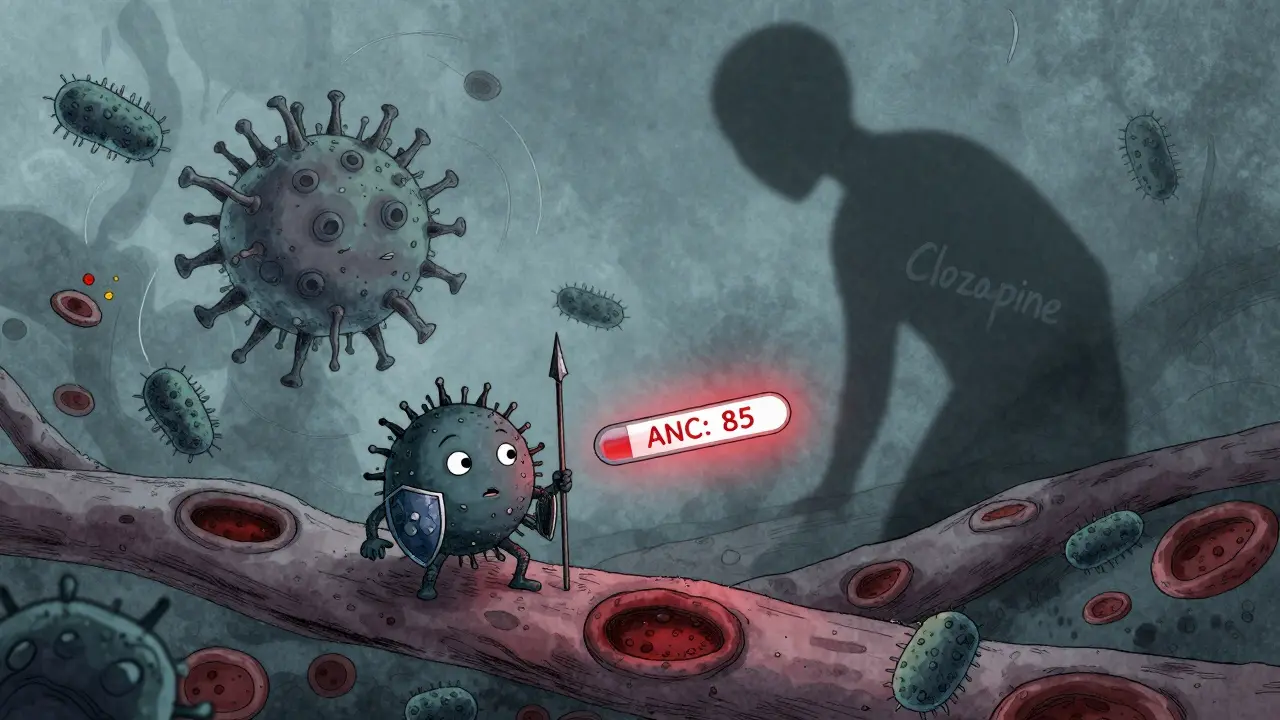
Medication-Induced Agranulocytosis: Infection Risks and Essential Monitoring Steps
Medication-induced agranulocytosis is a rare but deadly condition that destroys infection-fighting white blood cells. Know the high-risk drugs, recognize early symptoms, and follow strict monitoring rules to prevent fatal infections.

The Beers Criteria: Potentially Inappropriate Medications for Seniors
The Beers Criteria is a vital guide for identifying medications that may be unsafe for seniors. Learn which drugs to avoid, why they're risky, and how to work with your doctor to find safer alternatives.
Stability and Shelf Life: Understanding Generic Drug Degradation and Safety Risks
Understanding how generic drugs degrade over time and why shelf life matters for safety. Learn the science behind stability testing, real-world risks, and what patients can do to protect themselves.

Black Box Warnings: What You Need to Know About the FDA’s Strongest Drug Safety Alerts
Black box warnings are the FDA's strongest safety alerts for prescription drugs, signaling serious risks of death or injury. Learn what they mean, how they work, and what to do if your medication has one.

Biologic Patent Protection: When Biosimilars Can Enter the U.S. Market
Biologic drugs in the U.S. enjoy 12 years of exclusivity before biosimilars can enter. Complex patents, high development costs, and legal delays keep prices high and access low-especially for rare disease treatments.

Reassurance from Research: What Clinical Studies Say About Switching from Brand to Generic Medications
Clinical studies show that while most generic medications work as well as brand-name drugs, some-especially for epilepsy and heart conditions-can cause problems after switching. Learn what the data says and how to protect your health.





