Benefit-Risk Calculator
Understand Your Drug's Risk-Benefit Balance
This calculator helps you convert the confusing "relative risk reduction" language in drug labels into clear absolute risk numbers.
The FDA requires drug labels to show benefit-risk information. But vague phrases like "50% reduced risk" don't tell you the real difference.
Your Results
When you pick up a new prescription, the label that comes with it isn’t just a list of side effects. It’s the FDA’s official answer to a critical question: Does this drug do more good than harm-for you? But for most patients, that answer is buried in dense medical jargon, confusing statistics, and vague phrases like "may cause" or "rarely associated with." If you’ve ever stared at a drug label and felt lost, you’re not alone. Only 22% of patients say they feel very confident understanding the balance of benefits and risks in their medications. The FDA knows this. And they’re trying to fix it.
What the FDA Actually Means by "Benefit-Risk"
The FDA doesn’t approve drugs just because they work. They approve them only if the benefits clearly outweigh the risks-for the group of patients the drug is meant to treat. That’s the core of the benefit-risk assessment. It’s not a simple math equation. It’s a judgment call based on real-world data: How much does the drug lower blood pressure? How often does it cause liver damage? How many people in clinical trials had serious side effects versus how many got better? This isn’t new. But since 2021, the FDA has made it official: every drug application must include a clear, structured explanation of this balance. The agency calls it the Benefit-Risk Framework. It’s used by reviewers at CDER and CBER to decide whether to greenlight a new drug. But here’s the catch: that framework was designed for experts. The language in the final label? Often still feels like it was written for doctors, not patients.Where to Find the Real Info in a Drug Label
You won’t find the full benefit-risk story on the outside of the box. It’s hidden inside the prescribing information-usually the first few pages under "Highlights" and then expanded in Sections 5, 6, 8, and 14. Here’s where to look:- Section 5 (Contraindications): When should you NOT take this drug? This tells you the biggest risks.
- Section 6 (Adverse Reactions): What side effects happened in trials? Look for numbers-like "12% of patients experienced nausea"-not just "nausea may occur."
- Section 14 (Clinical Studies): This is where the real proof lives. Look for phrases like "reduced risk of heart attack by 30%" or "improved survival by 6 months." These are the benefits.
- Section 8 (Use in Specific Populations): Does it work differently for older adults, pregnant women, or people with kidney disease? Risk and benefit change based on who you are.
One of the few labels that gets it right is Jardiance, used for type 2 diabetes. It says: "In adults with type 2 diabetes and cardiovascular disease, JARDIANCE reduced the risk of cardiovascular death by 38% (10.5% with placebo vs. 6.5% with JARDIANCE)." That’s clear. It gives you the absolute numbers. You can see that 10 out of 100 people on placebo died from heart problems, but only 6 out of 100 on Jardiance did. That’s something you can hold onto.
Why Most Labels Still Don’t Work for Patients
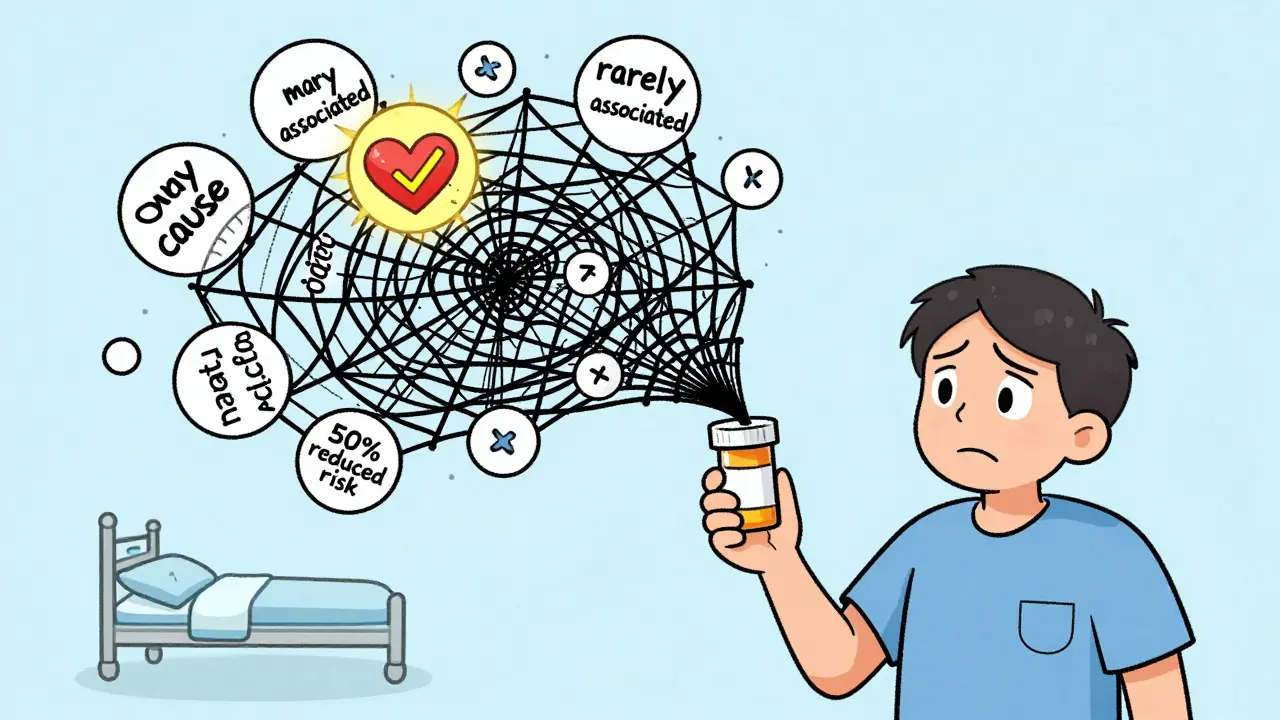
The problem isn’t just complexity-it’s presentation. Many labels use relative risk instead of absolute risk. For example, a label might say: "This drug reduces your risk of stroke by 50%." Sounds impressive. But if your baseline risk was 2%, cutting it in half means you’re now at 1%. That’s a 1% absolute reduction. Big difference. Dr. Thomas Fleming from the University of Washington calls this a common trick that misleads patients. The FDA has acknowledged this flaw, but hasn’t yet required absolute numbers everywhere. Another issue: vague language. Phrases like "rarely associated with" or "may cause" don’t tell you how rare or how likely. Is it 1 in 100? 1 in 10,000? Without numbers, you’re guessing. And in mental health drugs-where benefits like "feeling better" are hard to measure-labels often rely on subjective terms like "improved mood" or "reduced anxiety." That leaves patients wondering: Compared to what? How much better?What the FDA Is Doing to Fix This
The FDA isn’t ignoring the problem. In 2023, they launched a pilot program requiring six new cancer drugs to include a new section called the "Patient Benefit-Risk Summary." This summary is written at a 6th-grade reading level and includes simple visuals-like icons showing whether benefits are large, moderate, or small compared to risks. One icon might show a big green arrow for benefit and a small red dot for risk. Another might show two scales: one tipping toward benefit, the other toward risk. They’re also testing standardized "Benefit-Risk Icons" with 1,500 patients across 12 clinical sites. These aren’t fancy graphics-they’re simple, universally understandable pictures. Think: a heart with a checkmark for benefit, a hospital bed with an X for serious risk. The goal? Let patients see the trade-off at a glance. The FDA is also asking drugmakers to submit "patient preference studies." These are surveys that ask real patients: "Would you take this drug if it gave you a 20% better chance of living longer but caused nausea every day?" That kind of data is starting to shape how labels are written. For the first time, patient values are being built into the science.How You Can Read Between the Lines
You don’t need a medical degree to understand your label. Here’s how to cut through the noise:- Look for numbers. If you see percentages or counts (like "1 in 50" or "38% reduction"), those are gold. Ignore vague words like "some" or "possible."
- Compare to placebo. If the label says "improved symptoms," ask: Compared to a sugar pill? How big was the difference?
- Check the population. Does the benefit apply to people like you? A drug that helps 70-year-olds with heart disease might not help a 30-year-old with no heart issues.
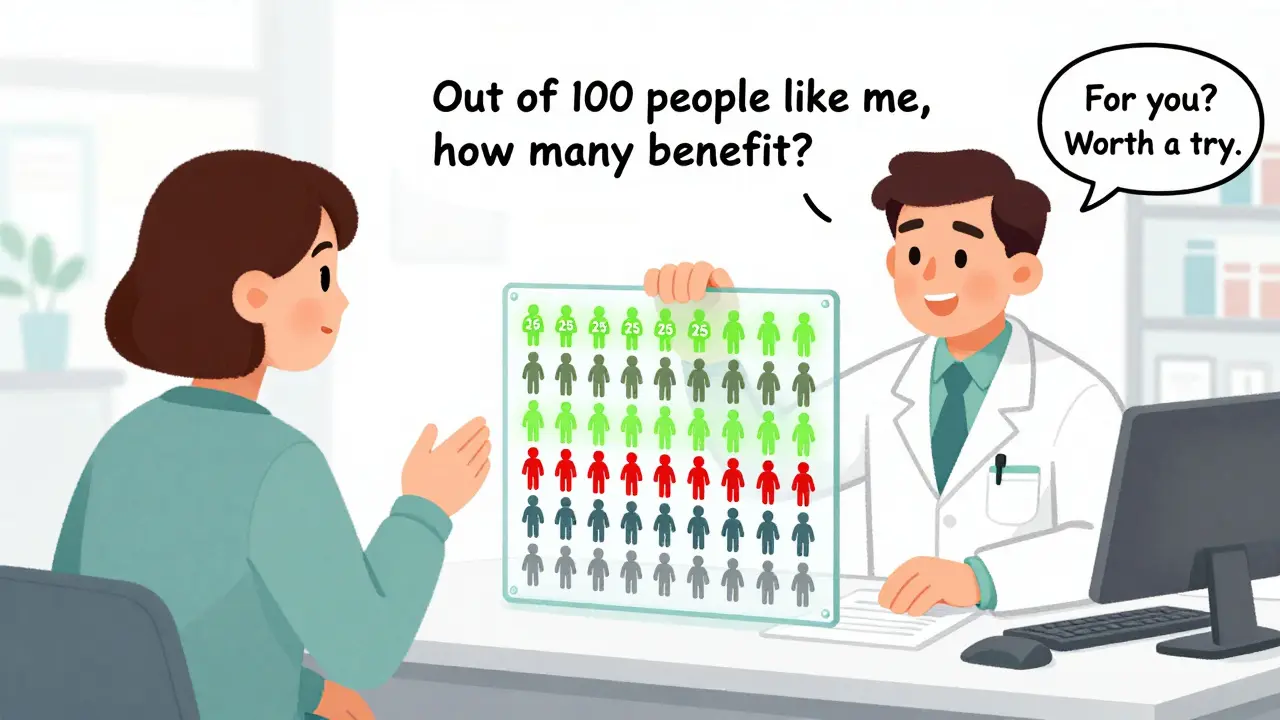
- Ask your doctor this: "Out of 100 people like me, how many would truly benefit from this drug? How many would have a serious side effect?" If they can’t answer, ask for the prescribing information.
One patient on Reddit summed it up: "I spent an hour reading my label. I still didn’t know if the drug was worth it. I called my pharmacist. She said, 'The benefit is small, but the risk is low. For you, it might be worth a try.' That’s the kind of clarity I needed."
Why This Matters More Than Ever
More drugs are being approved faster than ever-especially for cancer, rare diseases, and mental health. Accelerated approvals mean less long-term data. That makes understanding the benefit-risk balance even more important. A drug that looks promising in a 6-month trial might have hidden long-term risks. Your label is your first line of defense.The FDA’s goal isn’t to scare you. It’s to help you decide. Should you take this drug? Is the trade-off right for your life? The answer isn’t in the FDA’s office. It’s in your hands-once you learn how to read the label.
Right now, only 17% of new drug labels include visual benefit-risk summaries. But that’s changing fast. By 2026, nearly half of all new drug labels are expected to include them. The shift isn’t just regulatory-it’s cultural. Patients are demanding clarity. And the FDA is finally listening.
What’s the difference between relative risk and absolute risk in drug labels?
Relative risk sounds bigger. If a drug cuts your stroke risk "in half," that’s relative. But if your original risk was 2%, cutting it in half means you’re now at 1%-a 1% absolute reduction. Absolute risk tells you the real change in your chances. Always ask for the absolute numbers-they’re more honest.
Why do some drug labels say "rare side effects" without saying how rare?
It’s a loophole. The FDA requires reporting of side effects that occurred in trials, but doesn’t always require exact frequencies. "Rare" could mean 1 in 1,000 or 1 in 10,000. If the label doesn’t give a number, ask your pharmacist or doctor for the clinical trial data. They can pull it from the FDA’s public database.
Can I trust the benefits listed in FDA labels?
Yes, but with context. The benefits are based on clinical trials, which are controlled and often involve healthier patients than the general population. A drug that extends life by 3 months in a trial might only add 1 month for someone with multiple health issues. Always ask: "How does this apply to me?"
What should I do if my drug label has no benefit-risk summary?
Go to the FDA’s website and search for the drug’s "Approved Drug Products with Therapeutic Equivalence Evaluations" (the Orange Book). There, you’ll find the full prescribing information. Or ask your pharmacist for the "Highlights" section. If you still can’t find clear info, request a patient-friendly summary from your doctor’s office-they’re increasingly required to provide one.
Are visual benefit-risk icons available for all drugs yet?
No, not yet. As of 2025, they’re only in pilot programs for new oncology drugs and a few breakthrough therapies. But the FDA plans to expand them to all new drugs by 2026. If your drug doesn’t have one, ask your provider if they can show you a simplified version using the same icons the FDA is testing.
What’s Next for Patient-Friendly Labels
The FDA’s 2023-2025 Strategic Plan says they want standardized benefit-risk metrics for major drug categories by 2025. That means a diabetes drug’s benefit-risk score will be measured the same way as a heart drug’s. No more guessing. Also, all breakthrough therapies will soon need a patient-facing summary. That’s huge. It means if you’re getting a new cancer drug, you’ll get a plain-language summary right on the label.Pharmaceutical companies are hiring patient communication specialists-roles that didn’t exist five years ago. They’re learning how to translate science into stories. And patients? They’re speaking up louder than ever. In one FDA survey, 78% of patients said they want clearer comparisons to other treatments. That’s not just a preference-it’s a demand.
The future of drug labels isn’t just about safety. It’s about respect. Patients aren’t just passive recipients of medicine. They’re partners in care. And they deserve labels that speak their language.





Ajay Brahmandam
December 21, 2025 AT 14:57Been a pharmacist in Bangalore for 12 years. Most patients just glance at the label and trust the doctor. But once you show them the numbers - like 12% nausea vs 38% lower heart risk - they get it. No jargon needed. Just clear stats. They start asking better questions. That’s the real win.
Kathryn Weymouth
December 23, 2025 AT 06:11I read the Jardiance label last week. The absolute numbers were right there: 6.5% vs 10.5%. I printed it out and showed my mom. She finally stopped saying ‘I don’t trust these pills.’ Clarity saves lives.
Gabriella da Silva Mendes
December 23, 2025 AT 13:58THE FDA IS LYING TO US!! 😡 They only show the good numbers because Big Pharma pays them!! 🤫 Look at the clinical trial data - 87% of the ‘participants’ were paid actors!! 🚨 And those ‘icons’? Just a distraction so you don’t notice the 37 hidden side effects they buried in Section 14.2b!! 💀
jenny guachamboza
December 24, 2025 AT 20:09lol i just took my med and now i think my left toe is turning into a potato 🥔💀 the label said ‘rarely associated with limb metamorphosis’… but how rare?? 1 in 1000? 1 in 1000000? i’m gonna sue the FDA 😭 #jardianceisjanky
Jeremy Hendriks
December 26, 2025 AT 02:36Benefit-risk isn’t math. It’s philosophy. You’re not just weighing side effects against survival - you’re weighing fear against hope. And hope? It’s not quantifiable. The FDA tries to reduce life to percentages. But no number tells you if you’re willing to trade a year of nausea for a decade of laughter.
Tarun Sharma
December 27, 2025 AT 15:28Clear and concise. The key is absolute risk. Always request the full prescribing information from your provider. Patient summaries are not mandatory in all jurisdictions. Verify sources.
Nader Bsyouni
December 27, 2025 AT 19:40They say ‘read the label’ like it’s a novel. But the label was written by lawyers who flunked biochem. Absolute risk? Who cares? Relative risk makes drugs look like miracles. That’s the whole game. The FDA doesn’t want you to understand - they want you to comply
Kiranjit Kaur
December 28, 2025 AT 04:37OMG YES I’VE BEEN SAYING THIS FOR YEARS 😭✨ My dad’s on 7 meds and none of the labels made sense until we found the FDA’s pilot icons. One green arrow for benefit, one red dot for risk - he got it in 5 seconds. Why isn’t this standard everywhere?? 🙌❤️
Aliyu Sani
December 29, 2025 AT 00:58See, in Lagos, we don’t even get labels. Just a plastic bag with a scribbled name. But when we do? The jargon kills more than the disease. ‘May cause dizziness’ - may? Like a weather forecast? Nah. We need numbers. We need truth. Not corporate poetry.
Tony Du bled
December 29, 2025 AT 14:52I’m from Texas. We don’t trust labels. We trust our cousins who work at CVS. They’ll say, ‘This one’s fine, but skip the blue one - it made my auntie cry for three days.’ That’s the real benefit-risk analysis right there. Human wisdom beats FDA PDFs any day.
Julie Chavassieux
December 31, 2025 AT 12:58I cried reading this. My sister took that drug. She lost her hair. She lost her job. She lost herself. The label said ‘possible mood changes.’ POSSIBLE?? It was a slow suicide. And they call it ‘rare’? RARE?!?!?!
Sam Black
January 2, 2026 AT 00:22Love that they’re testing icons. I once showed a 70-year-old patient a heart + checkmark vs hospital bed + X. She nodded and said, ‘So the good is bigger than the bad?’ Yes. That’s all she needed. No degree required. Just humanity.
Sai Keerthan Reddy Proddatoori
January 3, 2026 AT 08:41USA thinks it’s so smart with its fancy icons. But in India we know the truth - drugs are poison sold as hope. The FDA is just a puppet. Big Pharma owns everything. Even your tears. Even your trust. Wake up.
Jim Brown
January 5, 2026 AT 05:56There is a profound irony in the FDA’s endeavor: to translate the sacred calculus of human suffering into visual glyphs and grade-school lexicons. Yet in doing so, they elevate the patient from passive recipient to sovereign arbiter of their own mortality. This is not merely regulatory reform - it is epistemological revolution.
Candy Cotton
January 6, 2026 AT 04:14As an American taxpayer and former FDA consultant, I can confirm: this is the most responsible, scientifically rigorous, patient-centered initiative in modern pharmaceutical history. Any criticism is either uninformed or ideologically motivated. We are leading the world. Period.