Autoimmune Risk Calculator
Enter your information and click "Assess My Risk" to get personalized insights.
Quick Takeaways
- Malabsorption can starve the immune system of crucial nutrients, prompting it to turn against the body.
- Conditions like celiac disease, Crohn's disease, and small‑intestine bacterial overgrowth are frequent bridges between digestive problems and autoimmunity.
- Leaky gut, altered gut microbiome, and vitamin D deficiency are the most common biochemical pathways linking the two.
- Targeted nutrition, gut‑healing protocols, and early medical screening can halt the cascade before irreversible damage occurs.
- Seek a gastroenterologist or an immunologist if you notice chronic diarrhea, unexplained fatigue, joint pain, or skin rashes together.
When you hear the phrase malabsorption, you might picture a stomach that just can’t break down a burger. In reality, it’s a complex chain of events happening mostly in the small intestine the primary site where nutrients pass from food into the bloodstream. If that passage falters, the immune system often perceives the shortage as a threat and can launch an attack against the body’s own tissues-what we call an autoimmune disorder a condition where the immune system mistakenly attacks healthy cells. Below we untangle the science, the symptoms, and the practical steps you can take today.
What Exactly Is Malabsorption?
Malabsorption is the body’s failure to extract and transport nutrients-vitamins, minerals, proteins, fats, and carbs-from the food we eat. The causes are diverse:
- Celiac disease an autoimmune reaction to gluten that damages the intestinal lining
- Chronic pancreatitis that reduces digestive enzymes
- Small‑intestinal bacterial overgrowth (SIBO) that competes for nutrients
- Inflammatory bowel diseases such as Crohn's disease a form of IBD that can affect any part of the gastrointestinal tract
The damage usually appears as villous atrophy-tiny finger‑like projections in the lining flatten, shrinking the surface area available for absorption.
How the Immune System Reacts to Nutrient Shortages
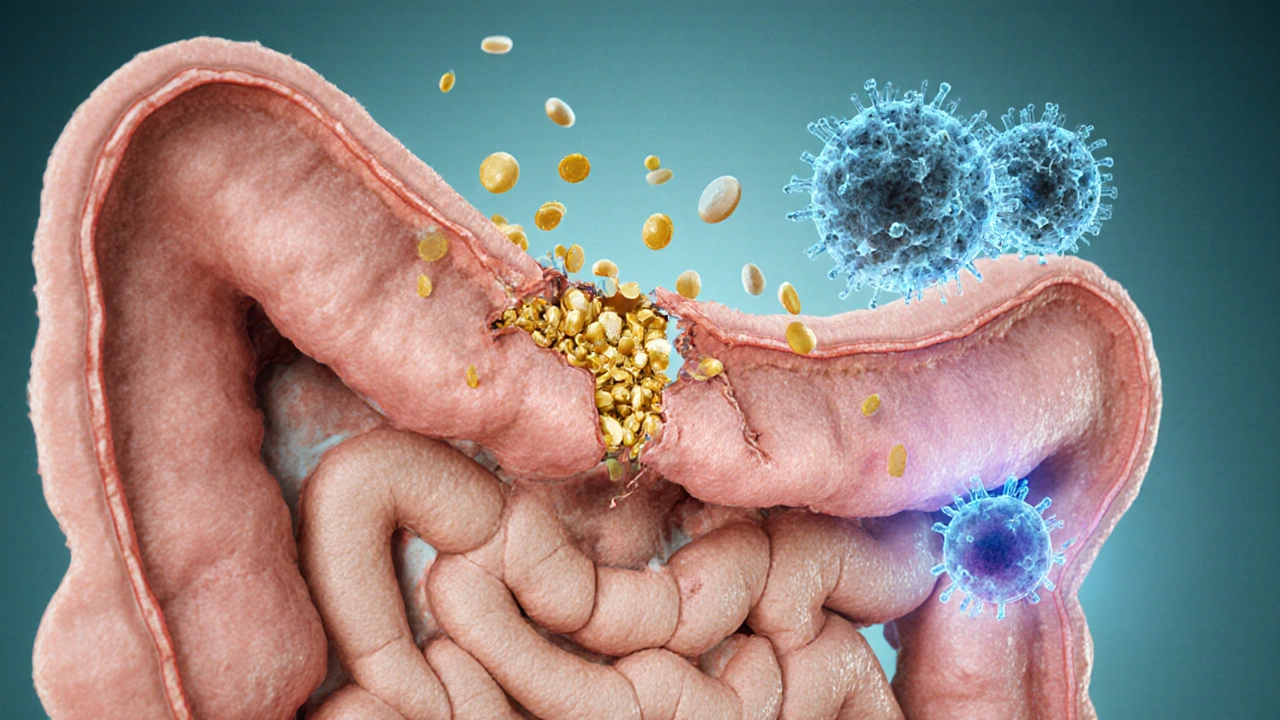
The immune system monitors blood levels of key micronutrients. Low vitamin D, zinc, or omega‑3 fatty acids can tip the balance toward inflammation. When the gut barrier becomes “leaky,” larger particles slip into the bloodstream, prompting an immune alarm.
Three biochemical pathways dominate the connection:
- Leaky Gut (Intestinal Permeability): Tight junctions between epithelial cells loosen, letting undigested proteins and endotoxins pass. The immune system tags these as foreign, producing autoantibodies that may later target similar structures in joints, skin, or thyroid.
- Microbiome Dysbiosis: A healthy gut microbiome the community of trillions of bacteria that live in the digestive tract trains immune tolerance. When bad bacteria dominate, they produce metabolites that stimulate inflammatory T‑cells, fostering autoimmunity.
- Vitamin D Deficiency: Vitamin D acts as a master switch for immune regulation. Malabsorption of fat‑soluble vitamins often leaves patients deficient, removing a crucial brake on autoimmune activity.
Key Autoimmune Disorders Linked to Malabsorption
Not every malabsorption case ends in autoimmunity, but several diseases show a striking overlap:
| Malabsorption Condition | Primary Autoimmune Link | Typical Biomarker |
|---|---|---|
| Celiac disease | Type 1 diabetes, autoimmune thyroiditis | Anti‑tTG IgA |
| Inflammatory bowel disease (Crohn's, ulcerative colitis) | Psoriasis, ankylosing spondylitis | C‑reactive protein (CRP) |
| Small intestinal bacterial overgrowth (SIBO) | Rheumatoid arthritis | Rheumatoid factor (RF) |
| Pancreatic exocrine insufficiency | Systemic lupus erythematosus (SLE) | Anti‑DNA antibodies |
Nutrition Strategies to Close the Leak
Repairing the gut and restoring nutrient uptake is a two‑pronged approach: eliminate the irritant and rebuild the barrier.
- Gluten‑free protocol: For anyone with celiac disease or gluten sensitivity, strict avoidance is non‑negotiable. Even trace exposure can reignite villous damage.
- Targeted probiotics: Strains such as Lactobacillus rhamnosus GG and Bifidobacterium longum have shown to reduce intestinal permeability in clinical trials.
- Prebiotic fibers: Inulin and resistant starch feed beneficial bacteria, encouraging short‑chain fatty acid production that tightens junctions.
- Vitamin D supplementation: Aim for 2,000‑4,000 IU daily, adjusting based on serum 25(OH)D levels (>30 ng/mL is optimal for immune modulation).
- Zinc and L‑glutamine: Both support enterocyte regeneration. A typical dose is 30mg zinc and 5g L‑glutamine two to three times per day.
Stay mindful of hidden sources. Processed foods often contain gluten a protein found in wheat, barley, and rye that can trigger celiac reactions even when the label says “natural.” Reading ingredient lists is essential.
Medical Tests to Pinpoint the Problem
If you suspect malabsorption, these investigations are the gold standard:
- Serology for celiac disease: Anti‑tissue transglutaminase IgA and total IgA levels.
- Stool fat test: Measures unabsorbed fat; >7g per stool suggests steatorrhea.
- Breath test for SIBO: Detects hydrogen or methane after a glucose or lactulose challenge.
- Endoscopy with biopsy: Direct visual of villous atrophy, especially useful for celiac or Crohn’s assessment.
- Serum nutrient panel: Vitamin D, B12, folate, iron, and zinc levels give a snapshot of absorption efficiency.
Results guide whether dietary changes are enough or if medication (e.g., pancreatic enzyme replacement, biologics for IBD) is required.
When to Seek Professional Help
Persistent symptoms-diarrhea, bloating, weight loss, chronic fatigue, joint pain, or a rash like dermatitis herpetiformis-should trigger a doctor’s visit. Early detection matters; the longer the gut stays inflamed, the higher the chance of permanent autoimmunity.
A multidisciplinary team works best: a gastroenterologist to evaluate digestion, a dietitian to craft a nutrient‑dense plan, and an immunologist to monitor autoantibody trends.
Bottom Line
Malabsorption isn’t just a digestive inconvenience; it can be the spark that ignites a full‑blown autoimmune cascade. By recognizing the signs, testing smartly, and repairing the gut barrier with targeted nutrition, you can break the cycle before it locks you into a lifelong autoimmune condition.
Frequently Asked Questions
Can malabsorption cause psoriasis?
Yes. When the gut barrier leaks, inflammatory molecules travel to the skin, triggering the hyper‑proliferation of keratinocytes seen in psoriasis. Treating SIBO or celiac disease often improves skin symptoms.
Is a gluten‑free diet enough to heal leaky gut?
For people with celiac disease, gluten removal is crucial and often restores gut integrity within months. For others, additional steps-probiotics, L‑glutamine, and vitamin D-are typically needed.
What blood test shows I have an autoimmune disorder linked to malabsorption?
Autoantibody panels vary by disease. Anti‑tissue transglutaminase IgA points to celiac, while anti‑thyroid peroxidase (TPO) indicates autoimmune thyroiditis. Discuss with your doctor which markers are relevant to your symptoms.
Can I take over‑the‑counter enzymes to fix malabsorption?
Enzyme supplements help when the pancreas isn’t producing enough lipase or protease (e.g., chronic pancreatitis). They don’t address immune‑driven villous damage, so pairing them with a gut‑healing protocol is essential.
How long does it take to see improvement after fixing my diet?
Most patients notice reduced bloating and better energy within 2‑4 weeks. Full villous recovery can take 6‑12 months, especially in severe celiac cases.







Dean Briggs
October 4, 2025 AT 00:32When we examine the cascade of events that begin with suboptimal nutrient absorption, we quickly realize that the gut is not merely a passive conduit but a dynamic, immunologically active organ that constantly dialogues with the rest of the body.
Every time a molecule of vitamin D or iron fails to cross the intestinal barrier, the body interprets this deficiency as a signal of stress, prompting immune cells to amplify their surveillance.
This heightened vigilance, while evolutionarily advantageous in the face of acute infection, can become maladaptive when sustained by chronic malabsorption.
Consider the role of the gut-associated lymphoid tissue (GALT), which houses a substantial proportion of our immune repertoire; when it perceives an ongoing breach in nutrient equilibrium, it may erroneously target self‑antigens, mistaking them for foreign invaders.
Moreover, the microbiota, which thrives on the substrates we digest, shifts composition when nutrients are scarce, fostering dysbiosis that further aggravates intestinal permeability, a phenomenon commonly referred to as "leaky gut."
Leaky gut permits translocation of bacterial components such as lipopolysaccharide (LPS) into the bloodstream, acting as potent inflammatory triggers that can tip the balance toward autoimmunity.
In parallel, the endocrine function of the gut, mediated by hormones like GLP‑1 and ghrelin, becomes dysregulated, influencing systemic metabolic pathways that intersect with immune regulation.
These hormonal perturbations often manifest as chronic fatigue and weight fluctuations, symptoms frequently observed in the early stages of autoimmune disease.
From a clinical perspective, patients presenting with unexplained joint pain or dermatological eruptions should be evaluated for underlying malabsorption syndromes, as correcting the absorptive deficit can sometimes blunt the autoimmune cascade.
Therapeutic strategies, therefore, extend beyond immunosuppression; they encompass nutritional rehabilitation, microbiome modulation, and restoration of barrier integrity.
Emerging research on targeted probiotic formulations and bioavailable micronutrient supplements offers promising avenues to recalibrate the gut‑immune axis.
In practice, a comprehensive assessment-incorporating stool studies, serologic markers of intestinal permeability, and dietary analysis-provides a roadmap for personalized intervention.
Ultimately, recognizing poor food absorption as a root cause rather than a peripheral symptom can transform our approach to preventing and managing autoimmune disorders.
By fostering collaboration between gastroenterologists, nutritionists, and immunologists, we empower patients to reclaim health from the cellular level.
It is a collective endeavor, one that hinges on the humility to acknowledge the gut's central role in systemic immunity.
Sadie Speid
October 9, 2025 AT 15:16This info is a game‑changer for anyone battling gut issues!
Sue Ross
October 15, 2025 AT 05:59I appreciate how the post connects the dots between nutrient deficiencies and immune hyperactivity.
It’s easy to overlook the gut when we talk about autoimmunity, but the evidence is mounting.
One practical takeaway is to get a comprehensive stool analysis before jumping into meds.
Also, monitoring vitamin D levels can give early hints about absorption issues.
Overall, a solid reminder to look deeper before prescribing broad immunosuppressants.
Rohinii Pradhan
October 20, 2025 AT 20:42While the premise is intriguing, the article neglects to cite peer‑reviewed sources that substantiate the mechanistic claims.
Statements about "leaky gut" often suffer from overgeneralisation and lack quantitative backing.
Furthermore, the suggested risk calculator appears rudimentary, ignoring confounding variables such as genetic predisposition.
In academic discourse, rigor demands more than anecdotal correlation; we need longitudinal studies.
Thus, the piece reads more like a promotional health blog than a scientific exposition.
Anna-Lisa Hagley
October 26, 2025 AT 10:26Your analysis lacks depth.
A Walton Smith
November 1, 2025 AT 01:09meh not convinced
Theunis Oliphant
November 6, 2025 AT 15:52Dean, while your enthusiasm is commendable, the narrative occasionally drifts into speculative territory without firm empirical footing.
For instance, the assertion that every micronutrient shortfall triggers GALT hyperactivity is an overstatement.
Nevertheless, your holistic perspective does prompt clinicians to consider gut health more earnestly.
I would recommend tempering the rhetoric with citations from reputable immunology journals.
India Digerida Para Occidente
November 12, 2025 AT 06:36Rohinii, I respect your insistence on rigor, yet we must also acknowledge the practical challenges of obtaining perfect data in everyday clinical settings.
The gut‑immune interface is inherently complex, and waiting for flawless evidence may delay necessary interventions.
Balancing scientific stringency with compassionate care is the art we strive for.
Therefore, integrating both perspectives can lead to more nuanced patient management.
Andrew Stevenson
November 17, 2025 AT 21:19Sadie’s point hits the nail on the head – the gut is a critical immunological organ.
From a functional medicine lens, we talk about the “gut‑immune axis” where dysbiosis and barrier dysfunction synergize to amplify systemic inflammation.
Key biomarkers like zonulin and LPS‑binding protein can help quantify permeability, guiding targeted therapy.
Moreover, employing a low‑FODMAP protocol alongside specific probiotic strains can restore microbial balance.
Incorporating these strategies into the risk calculator would enhance its clinical utility.
Overall, a more integrative framework bridges the gap between pathophysiology and actionable treatment.
Kate Taylor
November 23, 2025 AT 12:02Andrew, I love the jargon‑rich explanation – it really captures the complexity of the gut‑immune network.
Just a reminder that while advanced biomarkers are valuable, they’re not always accessible in primary care.
Simple dietary screenings can still flag at‑risk patients early on.
Keep pushing the conversation forward!
Hannah Mae
November 29, 2025 AT 02:46I think this is overhyped.
Iván Cañas
December 4, 2025 AT 17:29There’s merit to the gut‑autoimmunity link, but we must avoid a one‑size‑fits‑all narrative.
Culture, diet, and genetics all modulate how malabsorption impacts the immune system.
From my experience, patients with diverse backgrounds respond differently to the same nutritional interventions.
Thus, personalized assessments that respect cultural food practices are essential.
Balancing scientific evidence with cultural sensitivity ensures better adherence and outcomes.
Let’s keep the dialogue open and inclusive.
Jen Basay
December 10, 2025 AT 08:12Great overview! 😊 It really helped clarify how gut health ties into systemic immunity.
I’ve seen patients improve dramatically after addressing hidden malabsorption issues.
One tip: consider a trial of digestive enzymes before jumping to probiotics.
Also, don’t forget to test for H. pylori, as it can silently disrupt absorption.
Hope this sparks more holistic conversations in the community! 💡
Hannah M
December 15, 2025 AT 22:56👍 Absolutely love the emphasis on nutrition over meds! 🌱
When you fix the gut, the immune system often calms down on its own.
Keep spreading the word – the more people know, the better! 😃
Poorni Joth
December 21, 2025 AT 13:39This article reeks of pseudo‑science and gullible optimism.
Throwing buzzwords like "leaky gut" at readers without rigorous proof is irresponsible.
People need real, evidence‑based guidance, not feel‑good fluff that can delay proper diagnosis.
If you want to be taken seriously, stop pandering to the wellness market.
Facts matter more than anecdotes.
Yareli Gonzalez
December 27, 2025 AT 04:22I appreciate the balanced tone and the call for comprehensive testing.
It's crucial to empower patients with knowledge while respecting their individual journeys.
Thank you for the thoughtful perspective.
Alisa Hayes
January 1, 2026 AT 19:06The post does a solid job linking malabsorption to immune dysregulation, but a few clarifications could strengthen it.
First, specifying which micronutrients most commonly trigger autoimmune flares would add practical value.
Second, acknowledging that not all patients with gut issues develop autoimmunity helps avoid overgeneralisation.
Third, a brief note on the role of short‑chain fatty acids produced by the microbiota would round out the mechanistic picture.
Overall, it’s a commendable synthesis that invites deeper exploration.
Carl Mitchel
January 7, 2026 AT 09:49Delving into the intricate relationship between nutrient absorption and autoimmunity demands a nuanced, multidisciplinary approach that bridges gastroenterology, immunology, and nutritional science.
When the intestinal epithelium fails to efficiently absorb macro‑ and micronutrients, a cascade of physiological disturbances ensues, beginning with altered cellular metabolism within enterocytes.
These metabolic shifts can trigger the unfolded protein response, leading to endoplasmic reticulum stress and the activation of pro‑inflammatory transcription factors such as NF‑κB.
Concomitantly, the barrier function of the gut may become compromised, allowing antigens and microbial components to translocate into the lamina propria and systemic circulation.
This translocation acts as a potent stimulus for dendritic cells, which, in the context of a dysregulated cytokine milieu, may present self‑peptides to naïve T cells, breaking peripheral tolerance.
Furthermore, specific deficiencies-such as vitamin D, zinc, and omega‑3 fatty acids-have been implicated in modulating the Th17/Treg balance, tipping the scale toward auto‑reactive pathways.
Recent longitudinal cohort studies have demonstrated that patients with chronic malabsorption exhibit higher seroconversion rates for autoantibodies like anti‑CCP and ANA, reinforcing the clinical relevance of this mechanistic link.
Therapeutically, addressing malabsorption through targeted supplementation, enzymatic support, and microbiome modulation can restore barrier integrity and recalibrate immune homeostasis.
Emerging interventions, including butyrate‑producing probiotic strains and personalized nutrigenomic protocols, show promise in attenuating auto‑inflammatory trajectories.
In practice, a comprehensive assessment should include serum levels of key nutrients, stool analyses for dysbiosis, and functional tests of intestinal permeability such as lactulose‑mannitol ratios.
By integrating these diagnostics, clinicians can stratify patients based on their gut‑immune risk profile and intervene before overt autoimmune pathology manifests.
Ultimately, recognizing and correcting poor food absorption is not merely an adjunctive measure but a cornerstone in the prevention and management of autoimmune disorders.
Suzette Muller
January 12, 2026 AT 23:26Carl, your exhaustive overview is impressive and underscores the importance of a systems‑based perspective.
In my clinical experience, incorporating simple permeability tests alongside targeted nutrient panels often yields actionable insights.
It’s also vital to educate patients about food‑first strategies before resorting to immunosuppressants.
Thank you for highlighting the need for interdisciplinary collaboration.
Looking forward to more evidence‑backed guidelines in this space.